Fertilizer bombs typically use ammonium nitrate, NH4NO3. Bombers use it because the stuff is so ubiquitous and so plentiful.
Wiki draws a pretty picture of ammonium nitrate, which partially reveals its explosive potential:
Don't skip over that structure--it's a work of art: two very different nitrogen atoms separate elements of hydrogen and oxygen. On the left, four hydrogens surround a central nitrogen while on the right, three oxygen atoms surround a central nitrogen. There's an unbalanced symmetry. Those H's and O's would love to get together and quench, making water, leaving the naked nitrogens to couple, making N2 which is essentially air. The chemical structure of ammonium nitrate is a depiction of air separating water.
We never hear about spontaneous explosions of ammonium nitrate. Why is that? An old word from the Greek called stoichiometry answers why. Try and balance the chemical equation of NH4NO3 making N2 and H2O:
NH4NO3 => N2 + H2O
Look easy? I gave up. The reason is that the hydrogen to oxygen ratio is inherently 4 to 3 on one side and 2 to 1 on the other. No amount of tweaking the coefficients will balance that reaction. The stoichiometry just doesn't work.
This brings up just how fertilizer bombs do work, because they do make air and water from ammonium nitrate. There is always a little admixture of carbon (typically fuel oil) but charcoal would work too. The carbon combines with the "excess" oxygen present in NH4NO3, making CO2. This trick even has a name: cf. detonation vs. deflagration
Here's a question for any munitions experts out there: were ANFO's involved in Boston? I've heard that the differing sounds of detonation versus deflagration are diagnostic.
A second question is technical: is it possible (in theory) to isotopically label the nitrogen in manufactured ammonium nitrate and thus, after isotopic analysis of air samples at the bombing site prior to mixing, identify the source?
____________
[Added] Well this breaking news should cause a spike in ammo and food prices. The report says that water sprayed on NH4NO3 made it explode. That may actually have been heat from the fire or could have resulted from of what's called heat of hydration when a dry salt suddenly mixes with water. Some salts suddenly mixed with water actually make the water colder.
Ammonium nitrate (AN) is not known to chemically react with water in an explosive way. However, if heated it reacts explosively:
2NH4NO3 => 2N2 + O2 + 4H2O
Now that reaction really produces a sky-full: nitrogen, oxygen, & cloud.
Showing posts with label nitrogen. Show all posts
Showing posts with label nitrogen. Show all posts
Monday, April 15, 2013
Wednesday, April 4, 2012
Conversations with Henry
Henry: You forgot to mention Allen and Senoff.
Me: Who?
Henry: Allen and Senoff. They made the first dinitrogen complex from ruthenium, [Ru(NH3)5(N2)]2+. That news caused quite a stir back in 1965. Ruthenium rhymes with iron, so you're also speculating about molybdenum. It could be iron doing the fixing in nitrogenase. Never sell iron short.
Me: *gulps*
Henry: I'm glad to see that you're reading and keeping up with newer stuff. Barry told me you'd be back.
Me: Who?
Henry: Allen and Senoff. They made the first dinitrogen complex from ruthenium, [Ru(NH3)5(N2)]2+. That news caused quite a stir back in 1965. Ruthenium rhymes with iron, so you're also speculating about molybdenum. It could be iron doing the fixing in nitrogenase. Never sell iron short.
Me: *gulps*
Henry: I'm glad to see that you're reading and keeping up with newer stuff. Barry told me you'd be back.
Labels:
1965,
Conversations with Henry,
Conversations with Me,
iron,
Irony,
nitrogen,
Ruthenium
Molybdenum Fixes Nitrogen
Molybdenum's odd-sounding name comes from its resemblance to lead ores. That makes three elements: plumbum (lead), carbon (plumbago), and now molybdenum named after lead. Perhaps it's not so much the color as the texture:
Molybdenum's role in life is anything but leaden. Mo is in nitrogenase, an enzyme absolutely essential for life. Nitrogenase-containing bacteria "fix" nitrogen, turning thin air into solid plant food (ammonium), a feat no less remarkable than photosynthesis. The bacteria in turn get first dibs on the sugars made by the plants in perfect symbiosis.
If artificial photosynthesis is cool--making sugars and fuels from CO2--nitrogen fixation is even more exciting because we're about a third of the way there.* Bacteria make about 2/3 of the world's fixed nitrogen, and the ancient Haber-Bosch process does the rest. The Haber-Bosch process, first developed in 1913, enabled Germany to fight the First World War. Today it helps feed about a third of the world's population but it also consumes about 1-2 % of the world's annual energy supply. We can do even better, perhaps by learning from nature.
Nitrogenase manages to fix nitrogen under much milder conditions than Haber-Bosch, though like many natural processes, the yield is diffuse and unusable industrially. Molybdenum's connection to nitrogen fixation was first noted in 1930 when bacteria raised on ammonia showed no need for the element while those raised on nitrogen did (before the enzyme nitrogenase had even been identified). When nitrogenase was found, the next step was to crystallize it and determine its structure. This happened in 1992, at a time when molybdenum and the heavier tungsten were already famous for loosely binding things like H2 and N2 in synthetic tungsten compounds. Some thought perhaps nature had gotten there first. Alas, the structure gave few clues as to where and how nitrogen is activated, except that it happened stepwise, using protons and electrons instead of H2.
Another really cool thing about the nitrogenase X-ray crystal structure was the revelation of a mystery element "X" which could be either a C, an N, or an O atom. Several contact points surround the mystery element X which makes that a "Texas" atom. More recent work, published last year in Science, says it is carbon (link). More on that later.
______________
*There are vanadium-dependent nitrogenases which convert CO to alkanes--just like Fischer-Tropsch catalysts do: link That would be so cool if the X-factor were a Texas carbide.
 |
| Native ore: MoS2 |
 |
| Native plumbago (graphite) |
 |
| Native lead (Galena, PbS) |
If artificial photosynthesis is cool--making sugars and fuels from CO2--nitrogen fixation is even more exciting because we're about a third of the way there.* Bacteria make about 2/3 of the world's fixed nitrogen, and the ancient Haber-Bosch process does the rest. The Haber-Bosch process, first developed in 1913, enabled Germany to fight the First World War. Today it helps feed about a third of the world's population but it also consumes about 1-2 % of the world's annual energy supply. We can do even better, perhaps by learning from nature.
Nitrogenase manages to fix nitrogen under much milder conditions than Haber-Bosch, though like many natural processes, the yield is diffuse and unusable industrially. Molybdenum's connection to nitrogen fixation was first noted in 1930 when bacteria raised on ammonia showed no need for the element while those raised on nitrogen did (before the enzyme nitrogenase had even been identified). When nitrogenase was found, the next step was to crystallize it and determine its structure. This happened in 1992, at a time when molybdenum and the heavier tungsten were already famous for loosely binding things like H2 and N2 in synthetic tungsten compounds. Some thought perhaps nature had gotten there first. Alas, the structure gave few clues as to where and how nitrogen is activated, except that it happened stepwise, using protons and electrons instead of H2.
Another really cool thing about the nitrogenase X-ray crystal structure was the revelation of a mystery element "X" which could be either a C, an N, or an O atom. Several contact points surround the mystery element X which makes that a "Texas" atom. More recent work, published last year in Science, says it is carbon (link). More on that later.
______________
*There are vanadium-dependent nitrogenases which convert CO to alkanes--just like Fischer-Tropsch catalysts do: link That would be so cool if the X-factor were a Texas carbide.
Labels:
hypothesis,
Molybdenum,
nitrogen,
The Elements Series,
Tungsten,
Vanadium
Monday, March 12, 2012
"Don't call it transmutation. They'll have our heads off as alchemists"
The transmutation of elements was an ancient, discredited notion promulgated by alchemy (alchemy is to chemistry what astrology is to astronomy). Alchemists sought to turn base metals like lead into gold. They failed or were quacks and charlatans. And yet transmutation has always occurred naturally and has been practiced since 1917.
Natural transmutation was first discovered when Frederick Soddy, along with Ernest Rutherford, proved that radioactive thorium converted to radium in 1901. At the moment of realization, Soddy later recalled shouting out: "Rutherford, this is transmutation!" Rutherford snapped back, "For Christ's sake, Soddy, don't call it transmutation. They'll have our heads off as alchemists."
Transmutation became a fait accompli. But it was one thing to discover that atoms could naturally and spontaneously lose little pieces like an alpha particle or a beta particle or even a gamma ray. It was quite another thing to discover that atoms could add little pieces too.
In 1917, Rutherford projected alpha particles from radium decay through air and discovered a new type of radiation which proved to be hydrogen nuclei (Rutherford named these particles protons). Further experiments showed the protons were coming from the nitrogen component of air, and he deduced that the reaction was a transmutation of nitrogen into oxygen:
14N + α → 17O + proton
This was also the first demonstrative proof of artificial transmutation and the proton's existence. All that was needed now was the neutron which led to division and multiplication.
Natural transmutation was first discovered when Frederick Soddy, along with Ernest Rutherford, proved that radioactive thorium converted to radium in 1901. At the moment of realization, Soddy later recalled shouting out: "Rutherford, this is transmutation!" Rutherford snapped back, "For Christ's sake, Soddy, don't call it transmutation. They'll have our heads off as alchemists."
Transmutation became a fait accompli. But it was one thing to discover that atoms could naturally and spontaneously lose little pieces like an alpha particle or a beta particle or even a gamma ray. It was quite another thing to discover that atoms could add little pieces too.
In 1917, Rutherford projected alpha particles from radium decay through air and discovered a new type of radiation which proved to be hydrogen nuclei (Rutherford named these particles protons). Further experiments showed the protons were coming from the nitrogen component of air, and he deduced that the reaction was a transmutation of nitrogen into oxygen:
14N + α → 17O + proton
This was also the first demonstrative proof of artificial transmutation and the proton's existence. All that was needed now was the neutron which led to division and multiplication.
Labels:
1901,
1917,
alchemy,
nitrogen,
oxygen,
protons,
radioactivity,
Rutherford,
Soddy,
transmutation
Tuesday, February 7, 2012
Conversations with Henry
Henry: "Isoelectronic" is a perfectly fine concept. No need for you to feel it's inadequate. I'll give you an even easier example. We can play the same game with carbon, nitrogen, and oxygen:
[Henry sketches the Lewis structures for N2 and CO]:
Henry: Forget about the labels "C", "N", and "O" for a moment. Don't let them color your thoughts. Think of them as the numbers 12, 14, and 16:
Me: OK, but what does the little curved arrow 1p, 1n mean in your picture?
Henry: That's your little Maxwell's Demon, moving a proton and a neutron from one side to the other. There's no net loss or gain, but rather just a transfer.
Me: Are you pushing electrons too?
Henry: No! The electrons haven't moved yet but they feel the polarization: suddenly there's an extra charge on the oxygen side and one less charge on the carbon side. The electrons rearrange, being drawn slightly closer to the oxygen, but not completely, and the carbon, having less positive nuclear charge, is polarized negatively by the electrons. The molecules electron's are polarized like this:
Me: Ah, that explains why carbon monoxide binds to metals like iron in hemoglobin via its carbon.
[Henry sketches the Lewis structures for N2 and CO]:
Henry: Forget about the labels "C", "N", and "O" for a moment. Don't let them color your thoughts. Think of them as the numbers 12, 14, and 16:
Me: OK, but what does the little curved arrow 1p, 1n mean in your picture?
Henry: That's your little Maxwell's Demon, moving a proton and a neutron from one side to the other. There's no net loss or gain, but rather just a transfer.
Me: Are you pushing electrons too?
Henry: No! The electrons haven't moved yet but they feel the polarization: suddenly there's an extra charge on the oxygen side and one less charge on the carbon side. The electrons rearrange, being drawn slightly closer to the oxygen, but not completely, and the carbon, having less positive nuclear charge, is polarized negatively by the electrons. The molecules electron's are polarized like this:
Me: Ah, that explains why carbon monoxide binds to metals like iron in hemoglobin via its carbon.
Monday, February 6, 2012
Gallium Arsenide is Germane to Solar Cells
Gallium arsenide, a simple combination of two elements, interconverts light and electricity; GaAs lasers turn electricity into light and GaAs solar panels convert light back into electricity. There are alternative combinations of elements for these tasks, but each has its limits. What strikes me is how gallium and arsenic bookend germanium:
I need a name for "binary combination of elements which brackets and mimics another element." The term isoelectronic is close but doesn't cut it for me. There is a mathematical symmetry about GaAs in view of Ge and it goes like this: (31 + 33)/2 = 32 or, in chemical logic symbols: (Ga + As)/2 = Ge.
Like gallium arsenide, germanium is a photovoltaic material. Google "germanium solar cell" and you will find cutting edge research involving blends of gallium arsenide with germanium. I'm glad there is on-going research into new materials because I am not sure we should be putting arsenic on every rooftop much like we're putting mercury in every lightbulb.
A similar "bookend relation" occurs a couple rows up in the Table between boron, carbon, and nitrogen. Look how boron and nitrogen bracket carbon:
Once again, (5 + 7)/2 = 6. And just like carbon, boron nitride (BN) has both graphite- and diamond-like structures. One type of BN is even harder than diamonds: link
I see a pattern here: the centrality of the carbon group, C, Si, Ge, etc. to the family of main group elements:
I need a name for "binary combination of elements which brackets and mimics another element." The term isoelectronic is close but doesn't cut it for me. There is a mathematical symmetry about GaAs in view of Ge and it goes like this: (31 + 33)/2 = 32 or, in chemical logic symbols: (Ga + As)/2 = Ge.
Like gallium arsenide, germanium is a photovoltaic material. Google "germanium solar cell" and you will find cutting edge research involving blends of gallium arsenide with germanium. I'm glad there is on-going research into new materials because I am not sure we should be putting arsenic on every rooftop much like we're putting mercury in every lightbulb.
A similar "bookend relation" occurs a couple rows up in the Table between boron, carbon, and nitrogen. Look how boron and nitrogen bracket carbon:
Once again, (5 + 7)/2 = 6. And just like carbon, boron nitride (BN) has both graphite- and diamond-like structures. One type of BN is even harder than diamonds: link
I see a pattern here: the centrality of the carbon group, C, Si, Ge, etc. to the family of main group elements:
Labels:
Arsenic,
bloghetti carbonara,
Boron,
Gallium,
Germanium,
nitrogen,
periodicity
Monday, April 25, 2011
Saltpeter Is Not Rock Salt
If word histories made any sense, saltpeter should be rock salt. But instead rock salt is halite.
LL made a nice comment back here:
The Lewis structure of potassium nitrate invokes all that separated charge, which in a way depicts internal potential energy. Nitrates give explosions not just an oxidant (oxygen) but also nitrogen atoms badly wanting to make nitrogen gas. Whence the added oomph first discovered by the Chinese. The same principle is behind nitroglycerin (dynamite), trinitrotoluene (TNT), and nitromethane racing fuel: enhanced combustion.
I find nitrogen oxides intrinsically interesting and I even wrote a bit about them back here already; I mentioned impotence, but it's at the very end of the post. It's an odd coincidence that NO gets one hard while NO3- stands accused of the opposite.
 |
| Rock Salt (Halite) |
If word histories made any sense, saltpeter should be rock salt. But instead rock salt is halite.
LL made a nice comment back here:
I thought that you would take this thread in the direction of Potassium Nitrate (saltpetre), accused falsely of causing impotence in men and at the same time allowing gunpowder to go boom.
 |
| Saltpeter |
The Lewis structure of potassium nitrate invokes all that separated charge, which in a way depicts internal potential energy. Nitrates give explosions not just an oxidant (oxygen) but also nitrogen atoms badly wanting to make nitrogen gas. Whence the added oomph first discovered by the Chinese. The same principle is behind nitroglycerin (dynamite), trinitrotoluene (TNT), and nitromethane racing fuel: enhanced combustion.
I find nitrogen oxides intrinsically interesting and I even wrote a bit about them back here already; I mentioned impotence, but it's at the very end of the post. It's an odd coincidence that NO gets one hard while NO3- stands accused of the opposite.
Labels:
Chemistry of Sex,
LL,
nitrogen,
oxygen,
Saltpeter
Tuesday, March 1, 2011
Choke On This!
The word pnictogen refers, in Greek, to the concept of suffocation. The Germans even call nitrogen Stickstoff, meaning suffocating stuff. I choke a little myself on the word pnictogen every time I hear it -- it comes out sounding like feeble erudition. The neighboring group words chalcogen and halogen are only slightly better, and the latter at least finds widespread use. But the concepts pnictogen, chalcogen, and halogen are cool enough and neatly correspond to the idea of periodic rhyming of elements.
Nitrogen is the group leader for team pnictogen and I have arrived at element 15, phosphorus, in my little Aufmarsch from element 1 to element 112 or so. Link
Anybody got any good phosphorus stories?
Thursday, January 20, 2011
Buh-Bye NOxious Waste
Nitrogen oxides are everywhere. There are "good" ones and "bad" ones and they're all slightly different. A collection of pretty pictures is here: link Nitrogen oxides include laughing gas (good), nitrate and nitrite salts in foods (controversial), and nitrate plant fertilizers (good). Nitrogen oxides are even important in sex (good) because the molecule NO (nitric oxide) is key in sexual arousal.* Some nitrogen oxides do more harm than good: nitrogen oxides in clouds help form acid rain and the color of "brown cloud" comes from NO2. But worst of all, nitrogen oxides come out the tail pipes of more efficient diesel vehicles. I wrote about that back here.
The reason there are so many different nitrogen oxides is because nitrogen and oxygen are Periodic Table neighbors, and being so close together, each covets its neighbor's electrons when excited. But only when provoked. Air does not react with itself under normal circumstances. But give things some radiation, a spark, or a diesel motor, and O2 and N2 do the nasty together--they recombine:
N2 + O2 ---> 2NOx
There's an "x" subscript there because NO can react further to make NO2 and other nitrogen oxides. "NOx" is so noxious that the State of California forbade the sale of new diesel vehicles beginning in 2004 (I got one of the last VW Golf models in 2003). Daimler-Benz wanted to get those sales back (leave it to the Germans to invent all the new cool diesel technology) and Daimler's BlueTec technology returns NOx emissions whence they came--back to nitrogen and water:
4NO + 4NH3 + O2 ---> 4N2 + 6H2O
That equation is mass-balanced by the way, unlike-ahem, what one finds in the Internets (FTFY). The chemistry looks like complex gas-phase chemistry but actually a metal surface mediates the rearrangements (thankfully not another precious metal catalyst). In practice, NOx abatement injects ammonia into the exhaust stream upstream of the catalyst. Ammonia is the best "blue" additive in theory, but urea, which is more easily transported and handled, can also be used in Daimler's AdBlue technology. From what I've read and heard, the technology works as well but is very expensive. So what else is new. Owners are required to periodically charge up with fresh aqueous urea, which led one owner to ask whether he couldn't just "piss in a tank instead" hahah.
A good primer on how the technology works can be found here or GIY (Google it yourself)
____________________
*Has anyone noted the irony of "NO" (nitric oxide) being a key factor in male sexual arousal?
The reason there are so many different nitrogen oxides is because nitrogen and oxygen are Periodic Table neighbors, and being so close together, each covets its neighbor's electrons when excited. But only when provoked. Air does not react with itself under normal circumstances. But give things some radiation, a spark, or a diesel motor, and O2 and N2 do the nasty together--they recombine:
N2 + O2 ---> 2NOx
There's an "x" subscript there because NO can react further to make NO2 and other nitrogen oxides. "NOx" is so noxious that the State of California forbade the sale of new diesel vehicles beginning in 2004 (I got one of the last VW Golf models in 2003). Daimler-Benz wanted to get those sales back (leave it to the Germans to invent all the new cool diesel technology) and Daimler's BlueTec technology returns NOx emissions whence they came--back to nitrogen and water:
4NO + 4NH3 + O2 ---> 4N2 + 6H2O
That equation is mass-balanced by the way, unlike-ahem, what one finds in the Internets (FTFY). The chemistry looks like complex gas-phase chemistry but actually a metal surface mediates the rearrangements (thankfully not another precious metal catalyst). In practice, NOx abatement injects ammonia into the exhaust stream upstream of the catalyst. Ammonia is the best "blue" additive in theory, but urea, which is more easily transported and handled, can also be used in Daimler's AdBlue technology. From what I've read and heard, the technology works as well but is very expensive. So what else is new. Owners are required to periodically charge up with fresh aqueous urea, which led one owner to ask whether he couldn't just "piss in a tank instead" hahah.
A good primer on how the technology works can be found here or GIY (Google it yourself)
____________________
*Has anyone noted the irony of "NO" (nitric oxide) being a key factor in male sexual arousal?
Wednesday, November 17, 2010
Why doesn't the world just spontaneously combust?
Half of the recipe for world conflagration is the fuel--the other half is the oxygen. Thermodynamics say that we should burn up. So why don't we? Why don't we ignite like a puddle of gasoline or at least slowly rust away like a piece of iron? Rust never sleeps--ask Neil Young.
I threatened a while back here to explain why oxygen in the air doesn't spontaneously ignite with all the organic fuel on earth in one big conflagration. The short answer is a little matter of about 23 kilocalories of energy per mole needed for oxygen to react with most things. 23 kcals/mol is not a high barrier.
Spin provides why we abide (that's not a political jingle)
Molecular oxygen is chemically inert in the presence of hydrocarbons and carbohydrates because of the unusual nature of its electrons. It's really not hard to understand this uniqueness of oxygen if you've had even rudimentary chemistry. One trick is to consider it in light of nitrogen.
Nitrogen is oxygen's periodic table next door neighbor. Nitrogen is surrounded by five valence electrons, while oxygen is surrounded by six. If we put two atoms together, we get 10 and 12 valence electrons respectively. Lewis might have drawn N2 like this:
:N:::N:
The more usual Lewis structure for nitrogen is:
:N≡N:
Both Lewis structures predict a nitrogen-nitrogen triple bond between the atoms. [true chem nerds will appreciate that the bond order of three is understood as a single hot dog shaped sigma bond along the line connecting the atoms and two bun-shaped pi-bonds at right angles (orthogonal) to each other so as to not overlap. This is how 6 electrons can occupy space between atoms without violating Pauli's exclusion rule].
Now let's use our little Maxwell's Demon to go in and transmute each nitrogen atom into an oxygen atom, leaving everything else the same. We have to add two electrons to the picture because we added one proton on going from nitrogen to oxygen (never mind the neutron count for now). So we initially get something that looks like this:
:Ȯ≡Ȯ:
That Lewis structure predicts that there will be two unpaired electrons, one on each oxygen. In fact, oxygen is paramagnetic, having two unpaired electrons. But O2 doesn't have a triple bond--it has a double bond. Hmmm. What to do. We could simply dissolve one bond between the two atoms, giving two lone pairs on each atom:
:Ö=Ö:
This picture is consistent with the observed double bond in dioxygen. But that picture fails to account for the unpaired electrons in dioxygen. What is going here? Nothing less than the failure of simple Valence Bond Theory and Lewis' overly simplistic view of chemical bonding!
There is no acceptable Lewis structure for the garden variety dioxygen molecule.
If you've gotten through this far I'm afraid that I've led you into a little trap. The answer as to what is the electronic structure of O2 and why don't we spontaneously combust are in part given by what's called Molecular Orbital Theory, a theory which triumphed over Lewis' theory way back in 1930s.
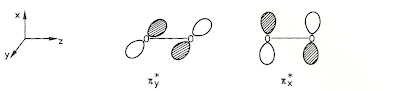
The two "extra" electrons in O2 aren't parked on individual atoms nor do they form a bond. Instead, they each reside in separate identical "beds," unpaired with each other. An oxygen molecule possesses two so-called antibonding orbitals which look like this:
One electron goes into the pi*y anti-bonding orbital and the other goes into an "orthogonal" pi*x anti-bonding orbital. Each orbital is singly occupied and electron is unpaired--just as observed. Oxygen still has a six bonding electrons--just like nitrogen does, but the two anti-bonding electrons partially negate the triple bond. The electrons tally like debits and credits, giving a net bond order of two as observed.
UV light can convert normal O2 into so-called so-called singlet oxygen which is drawn as:
:Ö=Ö:
When singlet O2 is loosed on organic material, all hell breaks loose, for example, sunburn and as a growing body of evidence shows, mutations and cancers.
Tuesday, August 31, 2010
Chemistry Is Like Sex: Coupling Illustrated
G.N. Lewis (I wrote about him back here) was from Berkeley and thus a bit more liberal when defining acids and bases: He gave more general definitions of them than Brønsted and Lowry did. And while more open-minded, Lewis was a bit of a chauvinist when he argued that a base's precious electrons helped "complete the octet" of an acid when they coupled.
Consider the coupling of a simple base, ammonia, with a simple Lewis acid, borane. If you're a jaded chemist who has "seen it all" you might consider just skipping to this link dealing with borane and ammonia making borazane as a hydrogen fuel energy source.
We already "know" what ammonia looks like here--but what about borane? I wrote a bit about boron the element back here. Turns out that the word "boron" is etymologically linked to the Arabic tongue as well, via the word borax.
Borane, BH3, is a natural fit for ammonia's lone pair. Consider its structure:
BH3 looks a bit like NH3 but completely lacks a lone pair. BH3 has only six surrounding electrons instead of eight and so is electronically unfulfilled. In a sense, it has a big hole in its middle. In the absence of an available lone pair, BH3 readily dimerizes in a head-to-tail fashion with another sister BH3 molecule to form B2H6. Here's an illustration of two BH3's getting it on together:
When NH3 and BH3 prepare to bond, a natural question is where should NH3 put its lone pair? BH3 has what's called a "virtual orbital" (there's nothing virtuous about it). A virtual orbital is just an empty electron orbital. Another name is a LUMO. Empty orbitals have metes and bounds, despite there being nothing there there. Here's a lurid depiction of borane's virtual orbital:
It's a bit hard to see in the depiction above but all three of borane's tripodal hydrogen limbs are squished flat into a planar configuration between the two swollen globes. The red and blue empty lobes are equivalent in the eyes of ammonia's incoming lone pair: Borane's empty orbital can be approached from above or below. As the ammonia approaches one side of borane, one empty lobe enlarges to accept the lone pair while the other shrinks. Also, borane's little hydrogen limbs fold back away from the incoming lone pair to accommodate the fit. The final coupling product looks like this:
Consider the coupling of a simple base, ammonia, with a simple Lewis acid, borane. If you're a jaded chemist who has "seen it all" you might consider just skipping to this link dealing with borane and ammonia making borazane as a hydrogen fuel energy source.
We already "know" what ammonia looks like here--but what about borane? I wrote a bit about boron the element back here. Turns out that the word "boron" is etymologically linked to the Arabic tongue as well, via the word borax.
Borane, BH3, is a natural fit for ammonia's lone pair. Consider its structure:
BH3 looks a bit like NH3 but completely lacks a lone pair. BH3 has only six surrounding electrons instead of eight and so is electronically unfulfilled. In a sense, it has a big hole in its middle. In the absence of an available lone pair, BH3 readily dimerizes in a head-to-tail fashion with another sister BH3 molecule to form B2H6. Here's an illustration of two BH3's getting it on together:
It's a bit hard to see in the depiction above but all three of borane's tripodal hydrogen limbs are squished flat into a planar configuration between the two swollen globes. The red and blue empty lobes are equivalent in the eyes of ammonia's incoming lone pair: Borane's empty orbital can be approached from above or below. As the ammonia approaches one side of borane, one empty lobe enlarges to accept the lone pair while the other shrinks. Also, borane's little hydrogen limbs fold back away from the incoming lone pair to accommodate the fit. The final coupling product looks like this:
BH3, with the help of ammonia's lone pair, now has an octet of electrons.
Labels:
Boron,
Chemistry,
Chemistry is like sex,
hypothesis,
nitrogen
The Basics Of How Chemistry Is Like Sex
Anyone who has cleaned with Windex has whiffed ammonia, a substance with a rich and interesting history that includes its very name: link. Note that ammonia was once called animal alkali. Muslims-in-science scholars should take note of the origin of the word alkali, but should take care not to get the concept of alkaline bases etymologically confused with that other Arabic word meaning base.
Ammonia gas easily condenses into a liquid when compressed (Albert Einstein and his erstwhile student Leó Szilárd once patented a refrigerator with no moving parts that used ammonia instead of freon). If Szilárd's idea had gone anywhere, he may not have bothered to have conceived the atomic bomb.
Ammonia has been variously depicted as NH3 or better as :NH3 or better still with its electron "lone pair" on full display, as:
Ammonia gas easily condenses into a liquid when compressed (Albert Einstein and his erstwhile student Leó Szilárd once patented a refrigerator with no moving parts that used ammonia instead of freon). If Szilárd's idea had gone anywhere, he may not have bothered to have conceived the atomic bomb.
Ammonia has been variously depicted as NH3 or better as :NH3 or better still with its electron "lone pair" on full display, as:
The lobe-like appendage sticking up is called a "lone pair" because there are two electrons in the orbital and because they're not associated with any atom except nitrogen. Some depictions of ammonia omit the lone pair but here I prefer the "fig leaf is off" depiction.
Ammonia is perpetually in search of an acid to quench its baser instincts. Given a proton like H+, ammonia and the proton instantly couple to make ammonium NH4+ in which all four H's become equivalent. In a real sense, the incoming acid polarizes the other three H's, sucking electrons away from them, making them all more acidic.
Ammonia is perpetually in search of an acid to quench its baser instincts. Given a proton like H+, ammonia and the proton instantly couple to make ammonium NH4+ in which all four H's become equivalent. In a real sense, the incoming acid polarizes the other three H's, sucking electrons away from them, making them all more acidic.
Labels:
Acids,
alchemy,
Chemistry is like sex,
Hydrogen,
hypothesis,
nitrogen
Sunday, May 30, 2010
Lewis Structures: What Is Essential Is Invisible
After physicists discovered and defined the basic properties of the naked electron, the next big question was how to describe and understand them in the context of atoms and molecules, thus encroaching the natural domain of chemistry.
G.N. Lewis invented Lewis structures as a way to describe and understand how electrons surround atoms and also how they hold molecules together. He did this in a non-mathematical, pictorial way in the early 20th century before the birth and subsequent ascent of quantum mechanics. Lewis depicted atoms and their electrons as cubes which could be joined at their edges, vertices, and faces:
The physicists regarded Lewis's theory as laughably crude, particularly the notion that electrons were fixed at certain positions. Lewis in turn was critical of the physicists' idea that electrons were completely fluid, as for example, in J. J. Thomson's plum-pudding model of the atom, because it seemed incapable of explaining the definitive shapes of molecules, for example, the tetrahedral geometry of carbon in countless organic compounds.
By the mid 1920's Lewis had dropped his 3D cubic portrayal of electronic structure; what survives today is rather like a flat 2D projection of those cubes onto a plane. Lewis would have drawn hydrogen cyanide (the molecule that may have killed him) as:
"What is essential is invisible to the eye"
The lasting importance of Lewis's theory is that it provided chemists with a way (albeit simplified) of visualizing the electronic structures of atoms and molecules. That is perhaps why it endures.
G.N. Lewis invented Lewis structures as a way to describe and understand how electrons surround atoms and also how they hold molecules together. He did this in a non-mathematical, pictorial way in the early 20th century before the birth and subsequent ascent of quantum mechanics. Lewis depicted atoms and their electrons as cubes which could be joined at their edges, vertices, and faces:
By the mid 1920's Lewis had dropped his 3D cubic portrayal of electronic structure; what survives today is rather like a flat 2D projection of those cubes onto a plane. Lewis would have drawn hydrogen cyanide (the molecule that may have killed him) as:
"What is essential is invisible to the eye"
The lasting importance of Lewis's theory is that it provided chemists with a way (albeit simplified) of visualizing the electronic structures of atoms and molecules. That is perhaps why it endures.
Monday, May 17, 2010
Maxwell's Demon
The Scottish mathematician James Clerk Maxwell introduced a useful little helper later dubbed Maxwell's Demon. Maxwell's demon could open and shut little windows for individual molecules and Maxwell used it to enable some thought experiments which violated the Second Law of Thermodynamics.
I need a nuclear equivalent to Maxwell's molecular demon: something that can go in and transmute elements into those one higher or lower in atomic number while leaving things like the electronic configuration alone. What I really want to do is to mentally change carbon atoms into boron atoms or carbon atoms into nitrogen atoms, or nitrogen atoms into oxygen atoms, all just to make a couple pedantic points:
Carbon - (1 proton, 1 neutron, & 1 electron) = Boron
or
Carbon + (1 proton, 1 neutron, & 1 electron) = Nitrogen
or
Nitrogen + (1 proton, 1 neutron, & 1 electron) = Oxygen
The new little demon can transmute dinitrogen into carbon monoxide, dinitrogen into dioxygen, and ethane into amino borane:
N2 ---> CO
N2 ---> O2
CH3CH3 ---> BH3NH3
I need a nuclear equivalent to Maxwell's molecular demon: something that can go in and transmute elements into those one higher or lower in atomic number while leaving things like the electronic configuration alone. What I really want to do is to mentally change carbon atoms into boron atoms or carbon atoms into nitrogen atoms, or nitrogen atoms into oxygen atoms, all just to make a couple pedantic points:
Carbon - (1 proton, 1 neutron, & 1 electron) = Boron
or
Carbon + (1 proton, 1 neutron, & 1 electron) = Nitrogen
or
Nitrogen + (1 proton, 1 neutron, & 1 electron) = Oxygen
The new little demon can transmute dinitrogen into carbon monoxide, dinitrogen into dioxygen, and ethane into amino borane:
N2 ---> CO
N2 ---> O2
CH3CH3 ---> BH3NH3
Labels:
bloghetti carbonara,
hypothesis,
nitrogen,
oxygen,
transmutation
Wednesday, April 28, 2010
Oxygen Reductio Ad Absurdum
Oxygen comes after nitrogen. The verb "to oxidize" connotes rusting, tarnishing, aging, and decay. What exactly does it mean to oxidize? One definition of oxidize clearly states "to turn something into an oxide." Here's the reductio ad absurdum for that definition (pun intended): Oxidized oxygen is oxide. That sentence is not true. Rather, reduced oxygen is oxide. In other words, when oxygen is oxidized we don't get an oxide as in the dictionary definition. There's no great mystery here: the common meanings for "to oxidize" and "to reduce" (chemically) were derived from what oxygen does to metals and not what happens to oxygen itself. Confusing? Read it again or move along.
Oxygen was unknown to the ancients but was present in all their classical elements: earth, wind, fire and water. Today we know that:
Unlike nitrogen, which preferentially* resides in the atmosphere, O2 is present in air solely due to bacteria, algae, and plants: they consume water and breathe in CO2, exhaling O2 and making food, essentially the reverse of what we do. Oxygen is pumped into the sky at a high energy cost (free energy-I'm tempted to say free will just to make a point). Atmospheric oxygen is like an enormous storehouse of chemical potential which works in tandem with chemically stored forms of energy like fossil fuels and plant stuffs. Oxygen in the air is in a sense one half of an enormous battery (the anode). A recent abstract explains:
P.S. That crazy-haired scientist is Prof. Martyn Poliakoff (I once had dinner at his house in Nottingham). Special thanks to Annie Gottlieb for first telling me about Martyn's series of chemistry videos.
______________________
* "Preferentially" is meant in the thermodynamic sense. Making N2 from nitride (or many reduced species is energetically favored whilst making O2 from oxide (water) is energetically uphill. Plants do this feat by harvesting sunlight.
Oxygen was unknown to the ancients but was present in all their classical elements: earth, wind, fire and water. Today we know that:
- in earth: rocks, sand, and dirt are mostly oxides.
- in wind: oxygen is about 20% of what we breathe.
- in fire: if there is no oxygen, there is no fire.
- in water: H2O.
Unlike nitrogen, which preferentially* resides in the atmosphere, O2 is present in air solely due to bacteria, algae, and plants: they consume water and breathe in CO2, exhaling O2 and making food, essentially the reverse of what we do. Oxygen is pumped into the sky at a high energy cost (free energy-I'm tempted to say free will just to make a point). Atmospheric oxygen is like an enormous storehouse of chemical potential which works in tandem with chemically stored forms of energy like fossil fuels and plant stuffs. Oxygen in the air is in a sense one half of an enormous battery (the anode). A recent abstract explains:
Through the photosynthetic action of cyanobacteria more than 2 billion years ago, dioxygen (O2) converted the earth’s atmosphere from a reducing medium to one that is powerfully oxidizing. As a result, we are now awash in a sea of chemical instability, literally ready at all times to combust to yield carbon dioxide and water (H2O). In other words, we are surrounded by enormous quantities of a gas that, from a thermodynamic point of view, is poised to react spontaneously with organic compounds and a wide variety of other reductants. While useful for generating heat, such reactions must be controlled if the oxidizing power of O2 is to be harnessed for the production of more tractable forms of energy and more complex (partially and selectively oxidized) chemical compounds. (link)The meeting abstract goes on to mention "spin":
Luckily, kinetic and spin barriers inhibit the direct reaction of O2 with organic materials and its reduction to H2O, extending the time we can exist in our current metastable atmospheric state.Explaining what is meant by "spin" nicely brings together the electronic structures of nitrogen, oxygen, and G.N. Lewis. It requires some additional chemical theory, but it's worth it to explain exactly why we don't all just spontaneously ignite (see next post). Anybody want to second guess me in the comments? Here's a hint: watch this YouTube video starting here; then back it up and watch the whole thing.
P.S. That crazy-haired scientist is Prof. Martyn Poliakoff (I once had dinner at his house in Nottingham). Special thanks to Annie Gottlieb for first telling me about Martyn's series of chemistry videos.
______________________
* "Preferentially" is meant in the thermodynamic sense. Making N2 from nitride (or many reduced species is energetically favored whilst making O2 from oxide (water) is energetically uphill. Plants do this feat by harvesting sunlight.
Wednesday, March 17, 2010
It's Amino World
The sky overhead is an ocean of nitrogen. Around 4,000 trillion tons of nitrogen (N2) float trapped up there because it's too light to sink back to earth and yet too heavy to float away into space (it's gravity shackled-unlike helium-the next lightest gaseous element). That enormous enveloping ocean has storms too. Nitrogen, and to a lesser extent oxygen, once set into motion, are essentially wind.
I once naively thought that azote, the French term for nitrogen, was etymologically related to the azure of a blue sky. Wrong! The aptly named azote literally means "no life" and that name was acquired shortly after oxygen and nitrogen were distinguished as the essential components of air: one gas supported life and one did not. The Germans call nitrogen Stickstoff, which literally means "suffocating-stuff". We call nitrogen "nitrogen" because it engenders "nitro" (nitro being an older word for saltpeter) which is an essential ingredient of gunpowder and plant fertilizer.
Despite the name azote, nitrogen compounds are quite lively and have a long and interesting history of blowing things up: gunpowder, dynamite, nitromethane racing fuel, TNT, and even airbags. In a very real sense, nitrogen's instability (chemical reactivity) under those circumstances is ultimately related to the stability of N2 and the element's propensity to get back to that elemental state, serenely floating above the fray.
Nitrogen is essential for all living organisms, which have tamed its volatility. Organic nitrogen is of course a constituent element of amino acids and thus of proteins, and also presents in nucleic acids (DNA and RNA). I wrote earlier about boron being fundamentally electron-poor with respect to carbon. Nitrogen, which sits on the right hand side of queen carbon, is the converse-electron rich with respect to the queen. That's really why nitrogen is an organic base.
Relatively little usable nitrogen is found in the earth. The amount in the air is about one million times larger than the total nitrogen contained in living organisms. Nitrogen availability is often the limiting factor in plant growth. Plants make organic nitrogen from air and we get usable nitrogen from plants (actually bacteria within the roots of some plants "fix" nitrogen to usable forms).
We no longer depend exclusively upon plants to make ammonia from the sky. During the First World War, Imperial Germany was cutoff from its sources of fixed nitrogen (mainly Chilean saltpeter and bat guano). Fritz Haber, the father of chemical warfare, developed the direct conversion nitrogen (N2) to ammonia (NH3) using hydrogen gas. Haber won the 1918 Nobel Prize in Chemistry for this feat, despite Germany losing the war and despite his wartime culpabilty in making things like chlorine and phosgene gases for trench warfare. The commercial Haber-Bosch process literally allowed the subsequent population bloom known as the Green Revolution. The process is still used today, highly refined, but essentially unchanged.
Subscribe to:
Posts (Atom)