Half of the recipe for world conflagration is the fuel--the other half is the oxygen. Thermodynamics say that we should burn up. So why don't we? Why don't we ignite like a puddle of gasoline or at least slowly rust away like a piece of iron? Rust never sleeps--ask Neil Young.
I threatened a while back here to explain why oxygen in the air doesn't spontaneously ignite with all the organic fuel on earth in one big conflagration. The short answer is a little matter of about 23 kilocalories of energy per mole needed for oxygen to react with most things. 23 kcals/mol is not a high barrier.
Spin provides why we abide (that's not a political jingle)
Molecular oxygen is chemically inert in the presence of hydrocarbons and carbohydrates because of the unusual nature of its electrons. It's really not hard to understand this uniqueness of oxygen if you've had even rudimentary chemistry. One trick is to consider it in light of nitrogen.
Nitrogen is oxygen's periodic table next door neighbor. Nitrogen is surrounded by five valence electrons, while oxygen is surrounded by six. If we put two atoms together, we get 10 and 12 valence electrons respectively. Lewis might have drawn N2 like this:
:N:::N:
The more usual Lewis structure for nitrogen is:
:N≡N:
Both Lewis structures predict a nitrogen-nitrogen triple bond between the atoms. [true chem nerds will appreciate that the bond order of three is understood as a single hot dog shaped sigma bond along the line connecting the atoms and two bun-shaped pi-bonds at right angles (orthogonal) to each other so as to not overlap. This is how 6 electrons can occupy space between atoms without violating Pauli's exclusion rule].
Now let's use our little Maxwell's Demon to go in and transmute each nitrogen atom into an oxygen atom, leaving everything else the same. We have to add two electrons to the picture because we added one proton on going from nitrogen to oxygen (never mind the neutron count for now). So we initially get something that looks like this:
:Ȯ≡Ȯ:
That Lewis structure predicts that there will be two unpaired electrons, one on each oxygen. In fact, oxygen is paramagnetic, having two unpaired electrons. But O2 doesn't have a triple bond--it has a double bond. Hmmm. What to do. We could simply dissolve one bond between the two atoms, giving two lone pairs on each atom:
:Ö=Ö:
This picture is consistent with the observed double bond in dioxygen. But that picture fails to account for the unpaired electrons in dioxygen. What is going here? Nothing less than the failure of simple Valence Bond Theory and Lewis' overly simplistic view of chemical bonding!
There is no acceptable Lewis structure for the garden variety dioxygen molecule.
If you've gotten through this far I'm afraid that I've led you into a little trap. The answer as to what is the electronic structure of O2 and why don't we spontaneously combust are in part given by what's called Molecular Orbital Theory, a theory which triumphed over Lewis' theory way back in 1930s.
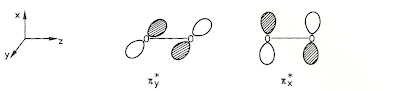
The two "extra" electrons in O2 aren't parked on individual atoms nor do they form a bond. Instead, they each reside in separate identical "beds," unpaired with each other. An oxygen molecule possesses two so-called antibonding orbitals which look like this:
One electron goes into the pi*y anti-bonding orbital and the other goes into an "orthogonal" pi*x anti-bonding orbital. Each orbital is singly occupied and electron is unpaired--just as observed. Oxygen still has a six bonding electrons--just like nitrogen does, but the two anti-bonding electrons partially negate the triple bond. The electrons tally like debits and credits, giving a net bond order of two as observed.
UV light can convert normal O2 into so-called so-called singlet oxygen which is drawn as:
:Ö=Ö:
When singlet O2 is loosed on organic material, all hell breaks loose, for example, sunburn and as a growing body of evidence shows, mutations and cancers.

Sometimes I just have to get things off my plate.
ReplyDeleteI feel your frustration with chemistry and with your inability to bend it to your will -- giving O2 a triple bond on a whim, or a single bond because you want to see what happens in real-time.
ReplyDelete@LL: Not my best effort. I'm highly distracted this week.
ReplyDelete